Improve your processes in the medical, pharmaceutical and healthcare sectors with SmartProcess
SmartProcess offers functions for process management, documents, SOPs – Standard Operating Procedures, workflow management, training and CAPA in the context of the requirements of “Good Working Practice (GxP)”. The functions of SmartProcess support the requirements of 21 CFR Part 11, Annex 11 to the EU GMP guidelines and ISO 13485 for medical device manufacturers. With SmartProcess as GxP software and SOP management software, you can improve processes in industries such as medical technology, pharmaceuticals and healthcare. You can model your processes and publish them with a release workflow.
As software for process management and quality management, SmartProcess optimally supports the requirements of the life sciences sector. To facilitate the validation of the GxP software in your company, we provide you with a document from the pre-validation of the release workflow, which controls the creation, review, release and publication of information and instructions.
SmartProcess as GxP software
Modeling and visualizing processes with the BPMN notation
Publish SOPs and documents with an approval workflow
Process and evaluate CAPA measures as a workflow
Recognize training needs and plan training courses
Coverage of the requirements of 21 CFR Part 11 with audit trail and electronic signature
In 21 CFR Part 11, the FDA defines requirements for the use of electronic records. Special requirements are also placed on the traceability of changes and electronic signatures. An audit trail is available for the traceability of changes. And in SmartProcess it can be set that the password is entered before an electronic signature. We would be happy to send you a white paper on how SmartProcess covers 21 CFR Part 11.

Process maps for structuring your processes
You can display the main processes in the GxP software and assign processes easily. The process maps provide users with a transparent overview of all processes. Process maps allow you to optimally recognize interactions between processes. SmartProcess provides you with convenient support in the GxP software for displaying main processes.
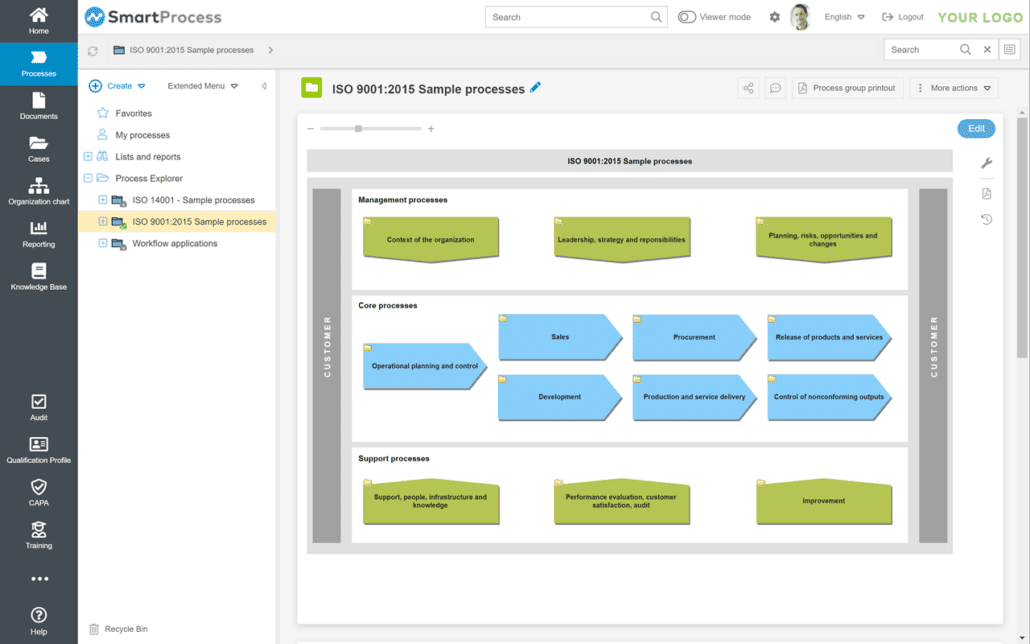
Modeling and publishing processes
Use the process management functions to model processes in the GxP software and SOP software. The process descriptions support you, for example, with the GxP requirements and with the coverage of ISO 13485. SmartProcess is modeled according to the BPMN notation, which provides a transparent overview of processes and responsibilities with the swimlane representation. SmartProcess offers you user-friendly and consistent modeling that provides you with many options for improving your processes.
You check and publish the processes via a release workflow. The processes are versioned throughout. As GxP software, SmartProcess offers numerous functions for process management and quality management.
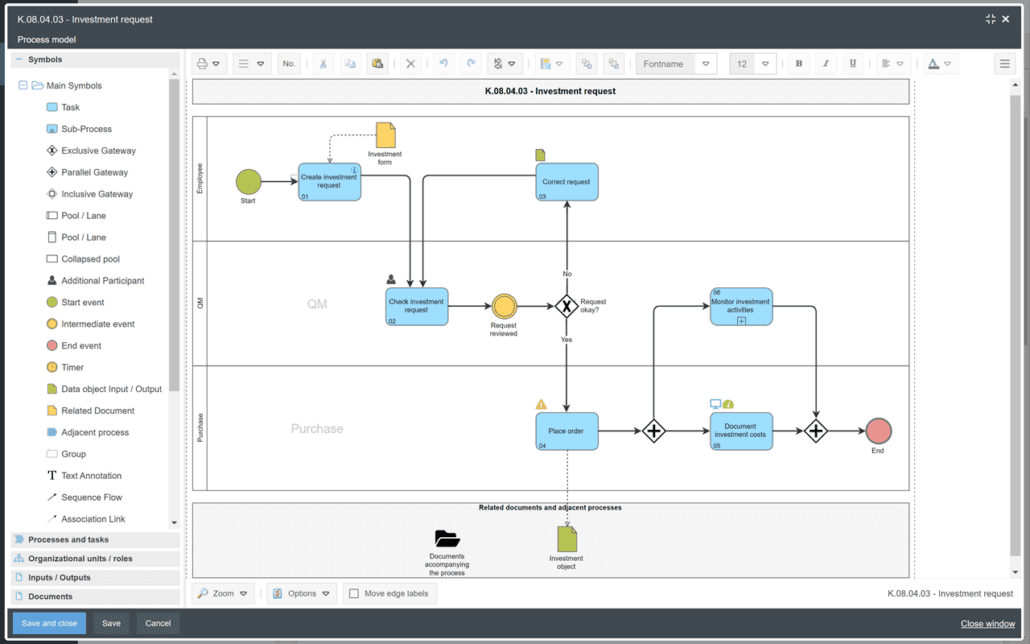
SmartProcess as document management and SOP management software
The documents are released and versioned in SmartProcess. This allows you to easily create Standard Operating Procedures (SOPs) and publish them with an approval workflow. As GxP software and SOP software, SmartProcess offers you many functions for document management. The documents are clearly marked with an automatically assigned number. Validity is also controlled automatically. You can edit documents directly via Check In / Check Out. Conveniently manage and publish documents in an audit-proof manner with SmartProcess.
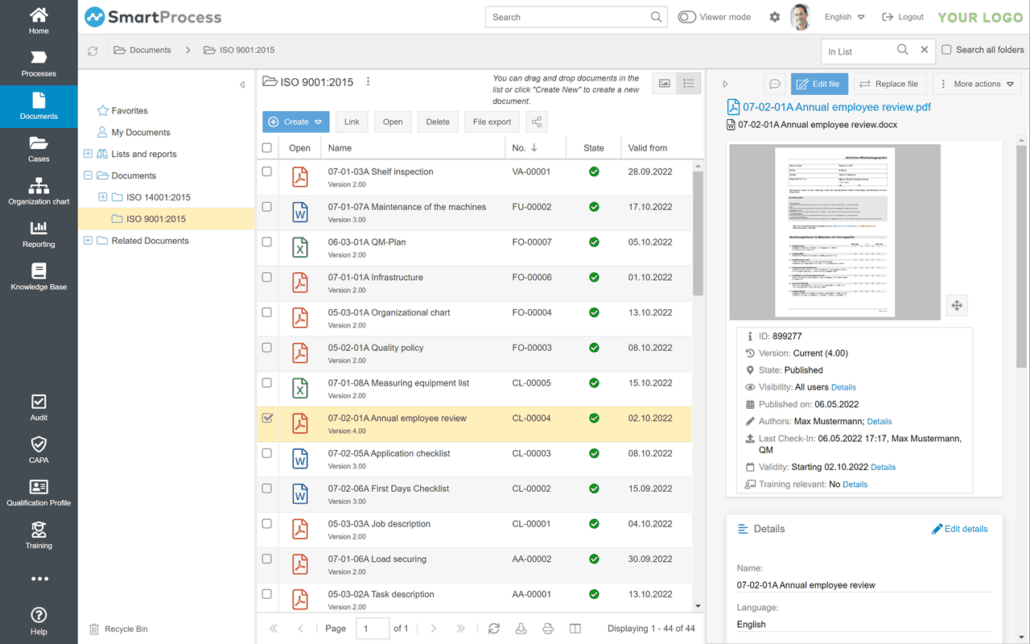
CAPA management
CAPA measures (Corrective Actions, Preventive Actions) are used to investigate deviations and initiate measures. The aim is to avoid new deviations and improve processes with corrective and preventive measures. SmartProcess enables you to record and control CAPA measures as a workflow, especially in the medical technology and pharmaceutical sectors. You can use the Workflow Designer in the GxP software to flexibly define the input masks and the processing sequence, and extensive reporting functions are available.
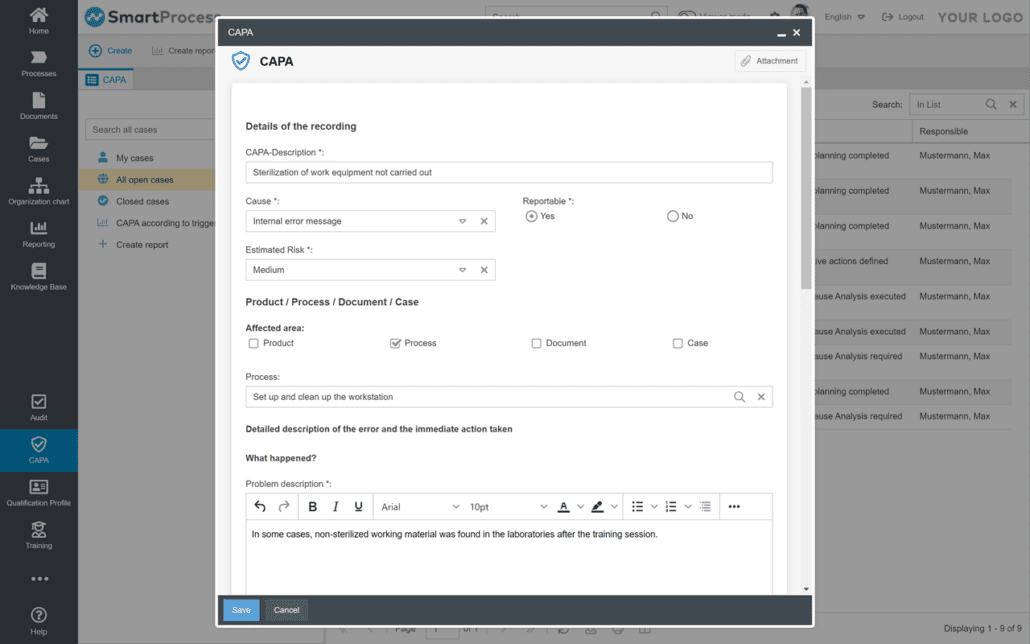
Automate processes with the GxP software using workflow functions
With the GxP software and SOP software SmartProcess for medical technology and pharmaceuticals, you can also automate the processing of procedures, forms and approvals. Input masks and processes can be flexibly defined. Open processes and tasks are displayed transparently in a task list. With the workflow functions, you can record a lot of data electronically and process it as a workflow.
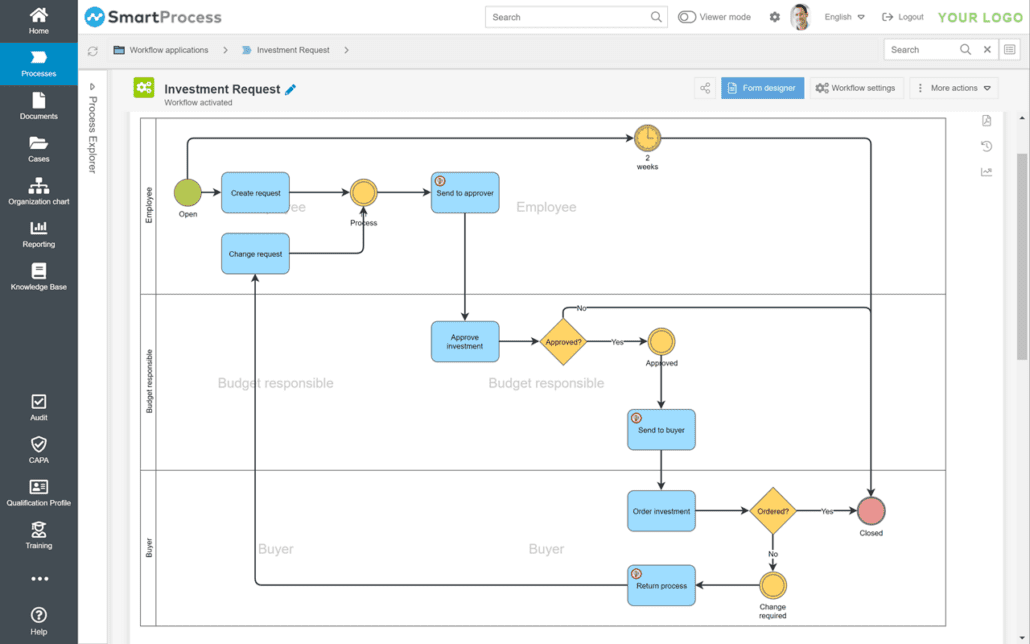
Qualifications and training
You can easily identify training needs with the GxP software SmartProcess. An employee’s qualifications and certificates are displayed in a qualification profile. The qualification matrix provides a transparent overview of all qualifications. You can plan training courses and integrate acquired qualifications from the training into the employee’s qualification profile. SmartProcess offers you end-to-end qualification and training management in the medical technology and pharmaceutical industries.
When processes or SOPs (Standard Operating Procedures) are published, they can automatically appear as target qualifications in the employee profile and can be confirmed there. Furthermore, a training or read confirmation is automatically requested from the employee when a publication is made. This ensures that employees are instructed and trained for new revisions.
Get to know SmartProcess - free of charge and without obligation
Further information